Clinical Evaluation
Classic Case
Clinical Evaluation Report (CER) Writing Service
- Categories:Clinical Evaluation
- Time of issue:2021-03-25 14:57:58
- Views:0
1. Definition of CER
According to the "Regulations on the Supervision and Administration of Medical Devices" and the "Administrative Measures for the Registration of Medical Devices" as well as the Chinese Good Clinical Practice, the medical device clinical evaluation refers to the process by which the registration applicant confirms whether the product meets the requirements for use through clinical study, clinical data, clinical trials, and other information.
2. The scope of application of CER
a)"The Guidelines for Clinical Evaluation of Medical Devices" are applicable to the clinical evaluation of Class II and Class III medical devices during the registration application, but not to the clinical evaluation of IVDs;
b)If technical guidelines for clinical evaluation of a specific product are released, the clinical evaluation of the corresponding product should follow relevant requirements.
3. Basic principles of CER
a)The clinical evaluation should be comprehensive and objective. Corresponding data should be collected through various means such as clinical trials. The clinical performance and safety data collected during the clinical evaluation process, as well as favorable and unfavorable data, should be included in the analysis.
b)The clinical evaluation should confirm the clinical use information, such as, the scope of application of the product (e.g., applicable population, applicable body part, contact method with human body, indications, degree and stage of disease, operating requirements, operating environment, etc.), application methods, contraindications, preventive measures, warnings, etc.
c)The registration applicant should draw the following conclusions through clinical evaluation:
i.Under normal use conditions, the product can achieve the expected performance;
ii.Compared with the expected benefit, the risk of the product is acceptable;
iii.The clinical performance and safety of the product are supported by appropriate evidence.
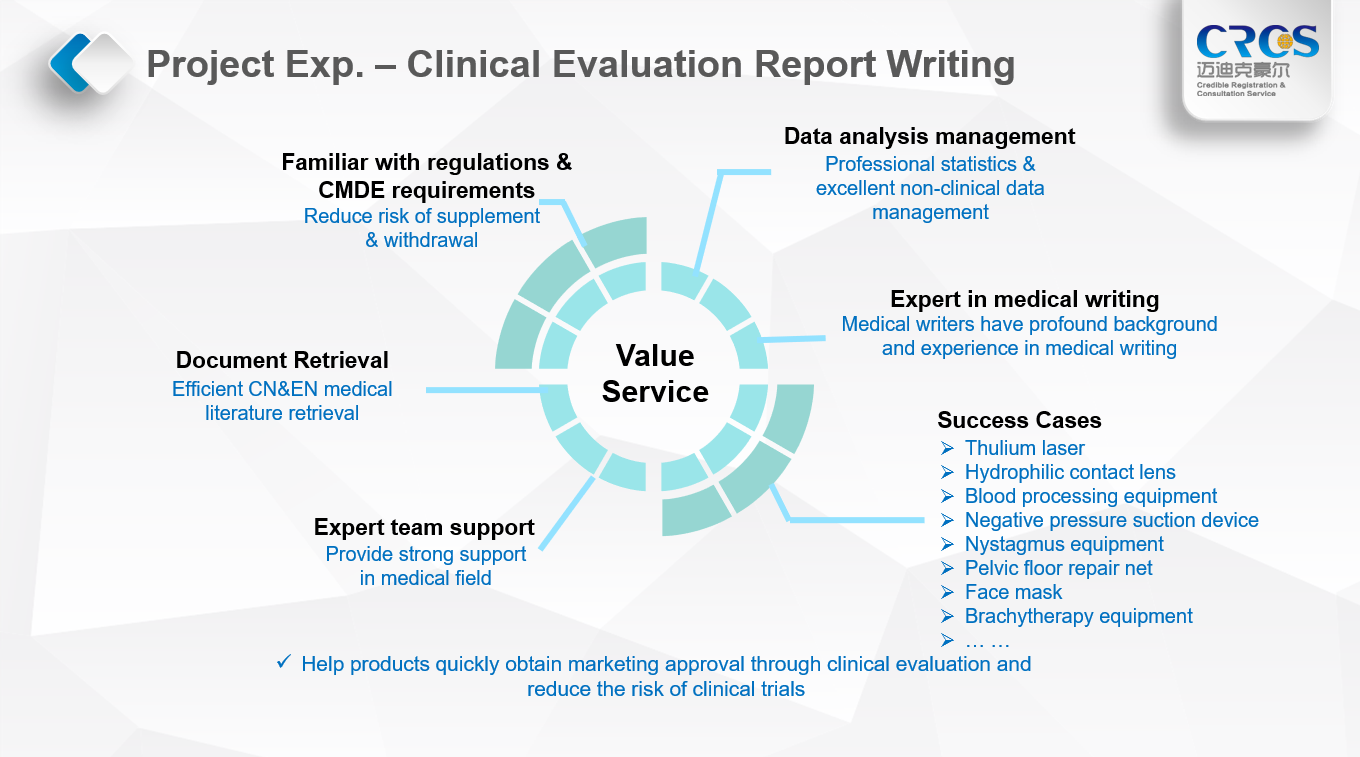
4. Premium services provided by CRCS
The preparation of CER for medical devices is a complicated process, which requires continuous communication with NMPA, and at the same time requires the cooperation between the client and CRCS to ensure the successful completion of the final report.
The clinical writing service is one of the quality services of CRCS. We provide clinical document writing services for customers who are going to carry out clinical trials and registration in China. It covers various medical documents, such as clinical evaluation report, clinical trial protocol, clinical trial report, medical report, etc. Furthermore, CRCS is competent to provide professional scheme design, clinical statistics and other clinical trial support for Hong Kong, Macao, Taiwan, and foreign customers who are preparing to enter the Chinese market.
Our employees of medical writing department have extensive clinical writing experience. Moreover, the abundant experience of medical device registration and clinical trial is a buttress for our clinical writing service. CRCS looks forward to providing guidance and help for your application and clinical research in China.
5. Distinguished advantages of CRCS
Scan the QR code to read on your phone


Telephones

info@crcs.com.cn

Address
Room 1009, Tower A, Longqin International Building, No. 168, Guang 'anmenwai Street, Xicheng District, Beijing
Company Introduction
The company specializes in medical device registration, in vitro diagnostic reagents registration, cosmetics agent service company, committed to the NMPA, CE regulation research and product registration and certification service has been more than 17 years.
Contact us
Beijing Medical & Care Co. Ltd.

