Team
- Categories:About us
- Time of issue:2021-03-19 15:01:35
- Views:0

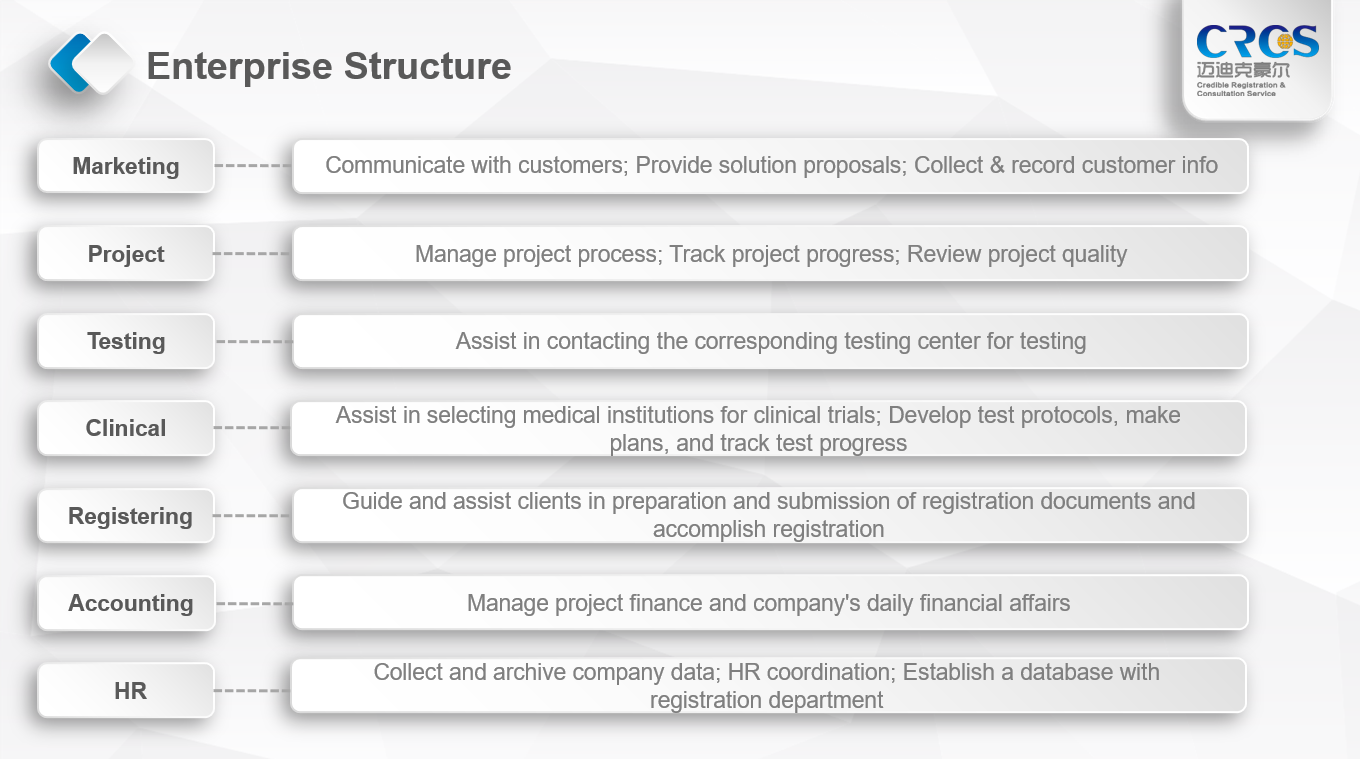
The company has a professional technical team that is familiar with product approval procedures and is well versed in national medical device regulatory laws and policies; it can provide professional one-stop services for global pharmaceutical companies. The company has cumulatively provided professional services to hundreds of companies around the world, completed thousands of related projects, and processed tens of thousands of documents, which was highly praised by customers. We will provide our customers with authoritative solutions with more efficient and better quality services, so as to help companies grasp the product and lead the opportunity to occupy the market.
In addition, the company has started to build a testing team since its establishment in 2002, and at the same time gradually conduct business dealings with Beijing, Tianjin, Jinan, Hangzhou, Shanghai and the China Inspection Institute for medical device and IVD product testing. There have been long-term inspection business communication and cooperation with the various inspection departments of the above-mentioned centers. Corresponding solution strategies and schemes can be given for the problems in the inspection. For the doubts and difficult issues in the inspection, we can assist in consulting related engineers or experts, and assist customers in solving problems. At present, 1000+ various inspections have been completed.
Scan the QR code to read on your phone


Telephones

info@crcs.com.cn

Address
Room 1009, Tower A, Longqin International Building, No. 168, Guang 'anmenwai Street, Xicheng District, Beijing
Company Introduction
The company specializes in medical device registration, in vitro diagnostic reagents registration, cosmetics agent service company, committed to the NMPA, CE regulation research and product registration and certification service has been more than 17 years.
Contact us
Beijing Medical & Care Co. Ltd.

